Home » Application » Batteries For Medical Devices
The Mission-Critical Power Behind Modern Healthcare
Hanery is a leading B2B manufacturer of high-reliability, safety-certified polymer lithium-ion batteries for the global medical device industry. From portable patient monitors to life-sustaining drug delivery systems, we engineer custom power solutions where performance and dependability are non-negotiable.
Safety
Low Self-Discharge
Long Lifespan
Lightweight
Your Expert Medical Battery Manufacturer in China
In an industry where every second counts, partner with a battery manufacturer that puts reliability first.
Uncompromising Safety & Compliance
We design and manufacture to meet the most stringent medical standards, including IEC 62133 and readiness for ISO 13485 quality systems.
High-Reliability & Long Cycle Life
Our batteries are built with premium materials and rigorous process controls for a long and predictable service life.
Full Traceability
We provide complete lot and component traceability, a critical requirement for medical device quality management.
Custom Engineering Solutions
We specialize in creating custom-sized, high-density batteries with intelligent BMS for complex medical devices.
Clean & Controlled Manufacturing
Our batteries are produced in a controlled environment to ensure the highest levels of purity and consistency.
Expert Regulatory Support
Our team has deep experience in navigating the complex world of global medical battery certifications.

Custom Medical Batteries as Your B2B Supplier
Hanery is your strategic partner for custom polymer battery packs, meticulously engineered for mission-critical medical applications. We understand that a medical battery is not just a component; it is a life-sustaining core. Our expertise lies in a risk-management-based design approach, ensuring every battery system we produce delivers flawless performance, communicates intelligently with the host device, and provides the ultimate peace of mind for both clinicians and patients.
Wholesale Medical Battery Applications
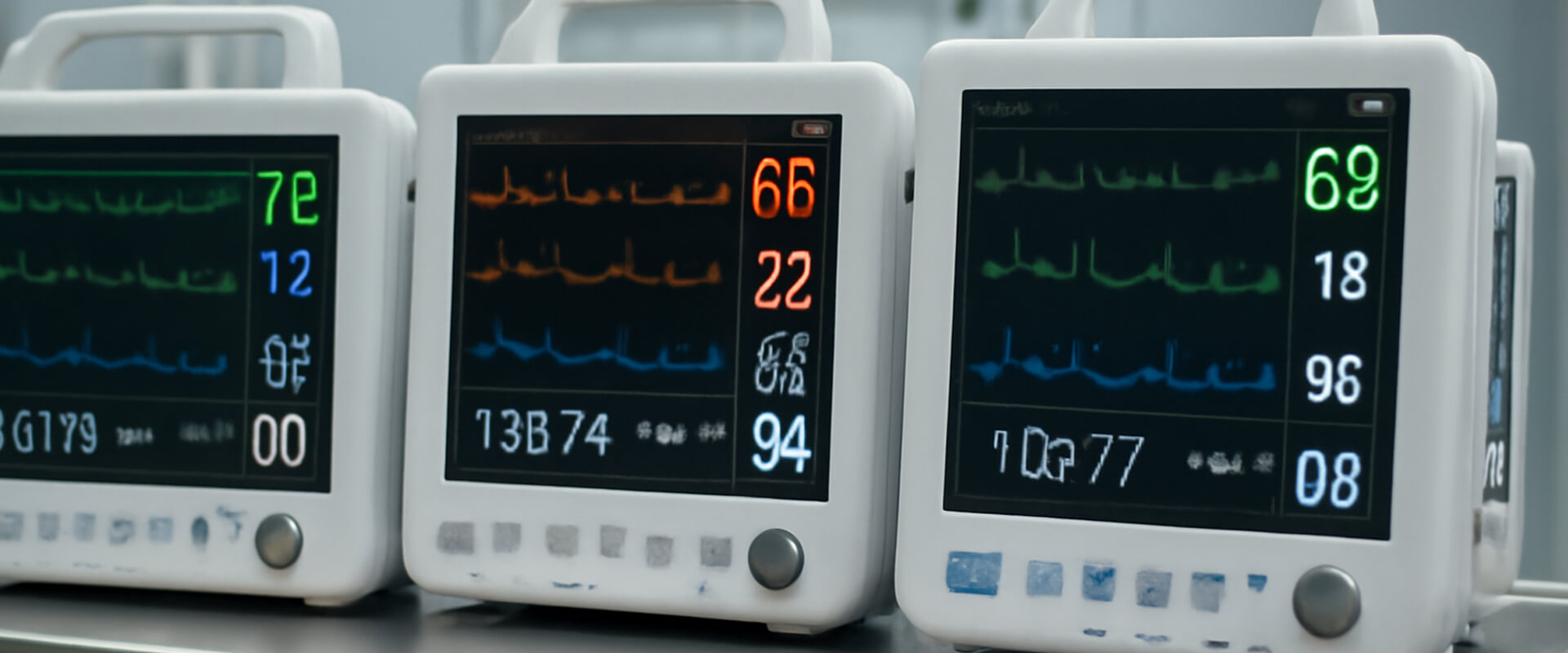
Portable Patient Monitors Batteries
Long-lasting, reliable battery packs for vital sign monitors, pulse oximeters, and portable ECG/EKG machines.
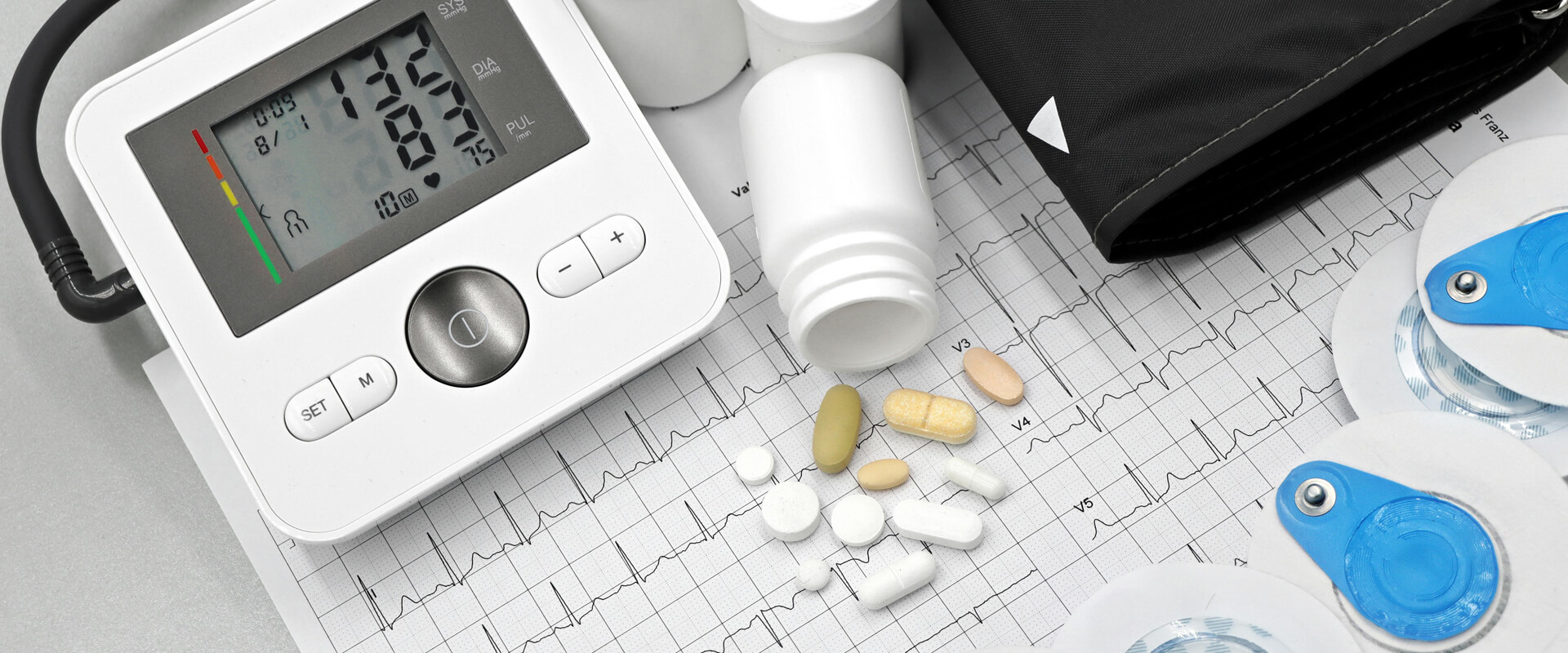
Drug Delivery Systems Batteries
Precise and highly reliable batteries for infusion pumps and insulin pumps where consistent power is critical.

Handheld Diagnostic Instruments Batteries
Compact, high-density batteries for portable ultrasound scanners, blood analyzers, and medical imaging devices.

Wearable Health Monitors & Hearing Aids Batteries
Miniature, custom-shaped, and safe rechargeable batteries for CGMs, hearing aids, and other body-worn sensors.

Portable Respiratory Devices Batteries
High-power, safety-certified battery packs for portable oxygen concentrators, nebulizers, and ventilators.
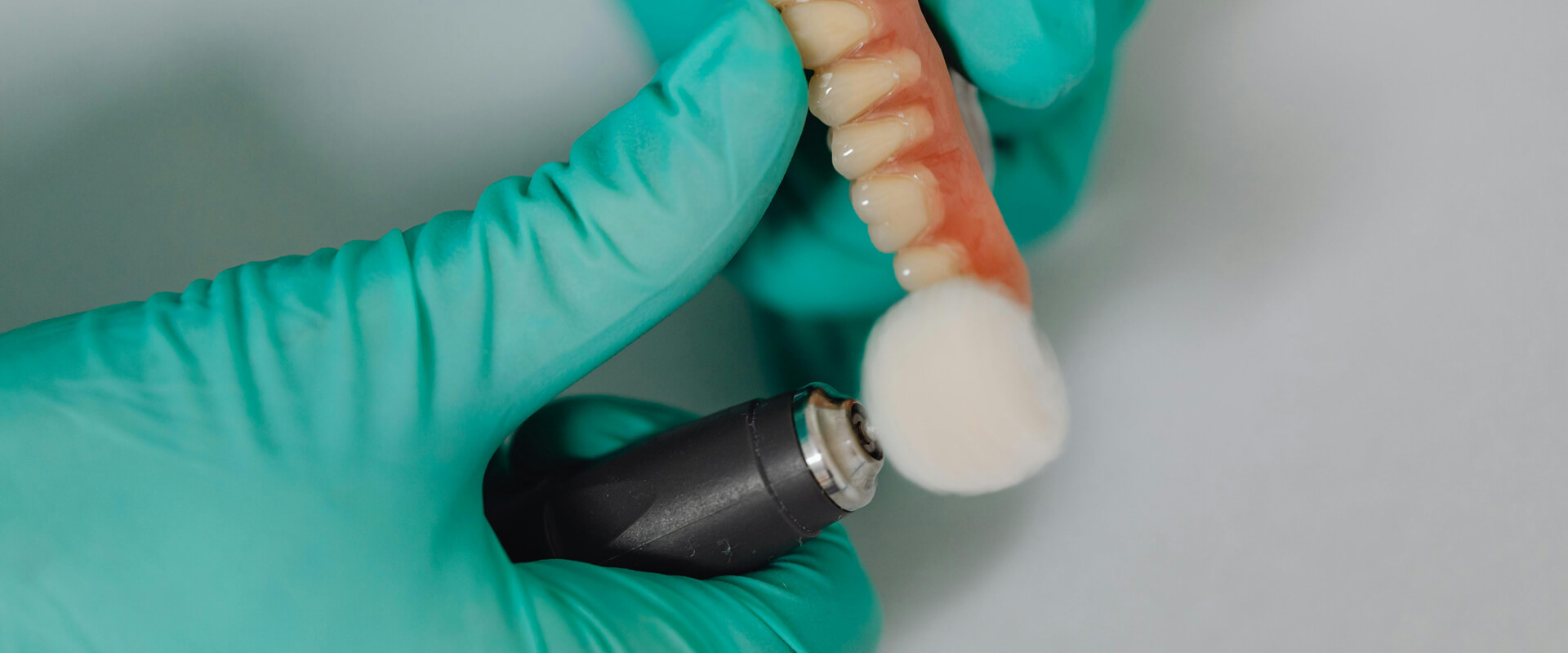
Surgical & Dental Tools Batteries
High-discharge-rate and sterilizable battery packs for cordless surgical drills, dental lights, and endoscopic cameras.

Powered Mobility & Prosthetics Batteries
Robust and durable battery systems for powered orthotics, advanced prosthetics, and patient mobility aids.

Hospital Equipment & Carts Batteries
High-capacity, swappable battery packs for mobile medical carts and critical equipment backup power.

Digital Medical Tools Batteries
Compact, reliable cells with long standby life for a new generation of smart medical tools like digital stethoscopes.
Navigating the Complexities of Medical Battery Certification?
Let our experts handle it. We provide comprehensive design, testing, and documentation support to ensure your product achieves global compliance.
Customized Options for Your Medical Batteries
We engineer every detail for clinical reliability and safety.
Application-Specific Chemistry
We select the optimal cell chemistry (e.g., LFP for safety/cycle life, high-density NCM for portability) for your device.
Intelligent BMS with Fuel Gauging
We integrate smart BMS with high-accuracy, redundant safety features and communication protocols for host device integration.
Custom Housing & Sterilization
We can design sterilizable enclosures and select materials compatible with medical cleaning protocols.
High-Reliability Connectors
We use medical-grade, high-mating-cycle connectors to ensure a secure and durable connection.
Redundancy & Fail-Safes
For life-sustaining devices, we can design battery systems with built-in redundancy and fail-safe mechanisms.
Unique Serialization & Traceability
Every pack is serialized for full traceability back to the cell batch and production data.
Get Your Batteries in Bulk, Step-by-Step
In-Depth Consultation & Risk Analysis
We analyze your device's power needs, use case, and regulatory requirements, performing an initial risk assessment.
System Design & DHF Support
We provide a comprehensive proposal with 3D models and can provide documentation to support your Design History File (DHF).
Prototyping & V&V Testing
We produce robust prototypes for your Design Verification and Validation (V&V) testing.
Certification Management & Approval
We manage the entire IEC 62133 and UN38.3 certification process and receive your final approval.
Controlled Mass Production
Your bulk order is manufactured under strict process controls (SPC) and quality checks.
Global Logistics & Lot Traceability
We manage the compliant shipping process, ensuring full lot traceability for every delivery.
FAQ for Medical Device Batteries
A: While our primary certification is ISO 9001, our quality management system is designed to be fully compliant with the principles of ISO 13485, and we have extensive experience supplying to ISO 13485 certified medical device manufacturers.
A: We use a multi-faceted approach: using only Tier-1 raw materials, 100% testing of all incoming cells, advanced BMS with redundant safety features, and a rigorous FMEA (Failure Mode and Effects Analysis) process during the design phase.
A: Due to the extensive engineering, documentation, and validation required, our MOQ for a new custom medical battery typically starts at 50,000 to 100,000 units.
A: The battery pack can be designed to be compatible with certain sterilization methods (e.g., EtO, gamma sterilization). Please specify your required sterilization method during the design consultation.
A: We provide a comprehensive documentation package for the battery system, including all test reports (like IEC 62133, UN38.3) and manufacturing quality data required for your device’s regulatory submission.
A: Our batteries are designed with low self-discharge rates. We can provide detailed storage guidelines and real-world data to ensure the battery meets your shelf life and performance requirements after storage.
A: Yes, we can design battery packs to meet intrinsically safe standards (e.g., ATEX or UL913) for specific applications. This requires a highly collaborative design process.
A: Given the stringent design and documentation requirements, the lead time for the first batch of medical-grade prototypes is typically 6-8 weeks.
A: Every battery pack receives a unique serial number that is tied to our Manufacturing Execution System (MES). This allows us to trace its entire history, from the raw material lots of the cells to the specific operators and test results during assembly.
A: Please provide your device’s power requirements (voltage, capacity, current), dimensional constraints, required certifications, use environment, and any specific safety or reliability targets.